What inside
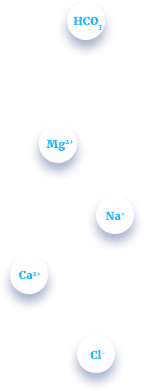
Mineral composition
Calcium+
30-70 mg/dm3
Calcium is an important determinant of water harness, and it also functions as a pH stabilizer, because of its buffering qualities. Calcium also gives water a better taste.
Magnesium
10-30 mg/dm3
Magnesium and other alkali earth metals are responsible for water hardness. Water containing large amounts of alkali earth ions is called hard water and converse.
Sodium
5-40 mg/dm3
The human body needs sodium in order to maintain blood pressure, control fluid levels and for normal nerve and muscle function.

Sulphate
10-50 mg/dm3
Sulphate is one of the major dissolved components of rain. High concentrations of sulfate in the water we drink is harmful, thereby we ensure a very low concentration in our mix.
Potassium
0.02-5
Potassium (K) is responsible for many other vital processes such as water and nutrient transportation, protein, and starch synthesis.
PH
6.5-8.5mg/dm3
Water with a pH level between 6 and 8.5 is safe to drink because it is neither acidic nor alkaline enough to be dangerous in the human body.